A systematic review is the first step in answering any research, policy, or practice question. Each systematic review follows certain steps. Systematic reviews and evidence synthesis are needed for Clinical Practice Guidelines, Clinical Evaluation Reports for Regulatory Approval, Health Technology Assessment (HTA), Health Economics and Outcome Research (HEOR), and Market Access, and before any Primary Research, Funding Application, Policy Making, and Clinical Decision-Making.
The only way to find the answer is to systematically find, assess, and synthesize all relevant data, answers, and literature, neither randomly nor selectively. There are more than 52 types of literature reviews with some ‘systematic’ components. The type of review you choose depends on your purpose, needs, time, and resources.
A systematic review is the only way to ensure you are not missing anything and that your work is novel and not a duplicate. Whether it is your personal work or a grant application, it starts with a systematic review, continues with one, and ends with it.
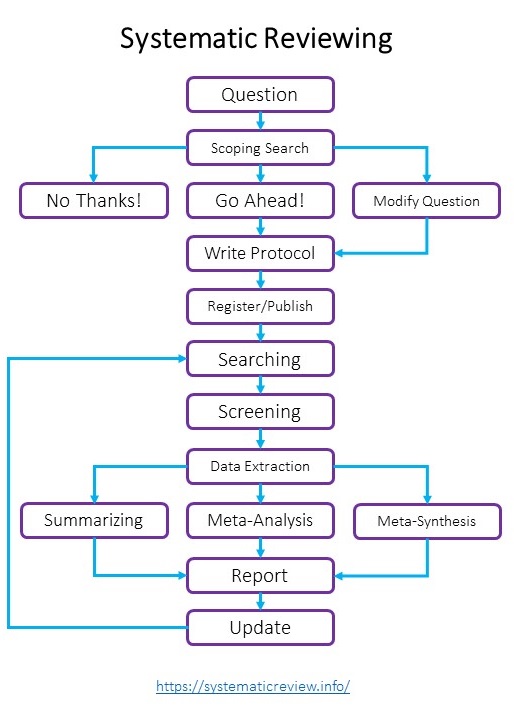
The above flow diagram shows the steps involved in the systematic reviewing process; however, not all literature reviews follow all these steps, and people usually tailor the process to fit their purpose.
If you have any questions or projects related to systematic or other types of reviews, please feel free to contact us through email or the contact form.
Updates
Chaptered Webinar Recording: How to Stay Up To Date in Your Field
Chaptered Webinar Recording: Responsible Use of AI in Evidence Synthesis: Filling the Gap Between Research and Practice? 30 October 2025